N,O-Dimethylhydroxylamine Hydrochloride: A Key Reagent in Modern Organic Synthesis
In the vast world of organic chemistry, there are reagents that steal the spotlight — flashy, reactive, and often dramatic in their transformations. Then, there are the quiet powerhouses: stable, reliable, and capable of facilitating key transformations that chemists rely on every day. One such unsung hero is N,O-Dimethylhydroxylamine hydrochloride, often abbreviated as DMHA·HCl.
Despite its small size and relative obscurity outside synthetic chemistry circles, DMHA·HCl plays a pivotal role in the synthesis of ketones and other important organic molecules. Its significance is especially pronounced in pharmaceutical and fine chemical manufacturing, where precision and selectivity are paramount.
🔬 What is N,O-Dimethylhydroxylamine HCl?
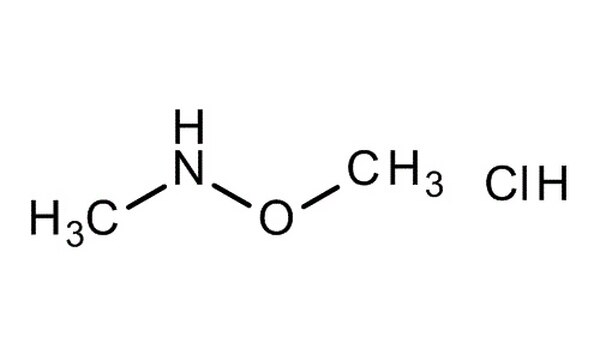
Chemically, N,O-Dimethylhydroxylamine hydrochloride is the hydrochloride salt form of N,O-dimethylhydroxylamine — a derivative of hydroxylamine where both the nitrogen and oxygen atoms are methylated.
-
Molecular formula: C₂H₈ClNO
-
Molecular weight: 97.55 g/mol
-
Structure: CH₃–N(OCH₃)H·HCl
The molecule contains two methyl groups: one attached to the nitrogen atom, and the other bonded to the oxygen. The hydrochloride component stabilizes the compound, making it easier to handle, store, and weigh compared to its free base.
This reagent is a colorless to off-white crystalline solid, typically supplied in high purity for laboratory and industrial use.
⚗️ A Cornerstone in Ketone Synthesis: The Weinreb Amide Route
The true value of DMHA·HCl lies in its use as a precursor to Weinreb amides, which are incredibly useful intermediates in organic synthesis. Weinreb amides were first introduced by chemist Steven Weinreb in the early 1980s and quickly became essential tools in controlling nucleophilic additions to carbonyl compounds.
Here’s why that matters:
In a typical Grignard or organolithium reaction with esters or acid chlorides, there’s a risk of over-addition. Instead of stopping at a ketone, the reaction can continue to form a tertiary alcohol, which may be undesirable.
But if you convert a carboxylic acid or acid chloride into a Weinreb amide using N,O-dimethylhydroxylamine, the addition of a Grignard or organolithium reagent produces only the ketone — no overreaction, no side products.
This precision has made the Weinreb amide route a go-to method in the synthesis of pharmaceuticals, natural products, and complex building blocks.
🧰 Applications in Research and Industry
N,O-Dimethylhydroxylamine HCl isn’t just limited to the lab bench — its utility spans multiple sectors:
1. Pharmaceutical Chemistry
In drug development, synthetic chemists often need to introduce ketone groups selectively without modifying other sensitive functional groups. DMHA·HCl makes this possible by enabling clean, predictable transformations using Weinreb amide chemistry.
2. Agrochemicals
Compounds used in herbicides and pesticides often require careful modification of carbon frameworks. DMHA·HCl is used to control acylation and alkylation steps in their synthetic pathways.
3. Material Science
In polymer and specialty materials research, controlling functional groups is crucial. N,O-Dimethylhydroxylamine derivatives are sometimes used in crafting polymers with precise molecular architectures.
4. Fine Chemicals and Intermediates
Used to synthesize dyes, flavors, and fragrances, DMHA·HCl helps create highly pure intermediates that might otherwise be difficult to isolate.
🛠️ Handling and Storage
While N,O-Dimethylhydroxylamine HCl is considered relatively safe compared to more reactive reagents, proper lab practices must still be followed:
-
Store in a cool, dry place, away from moisture.
-
Avoid inhalation, ingestion, or skin contact.
-
Use gloves, goggles, and a lab coat when handling.
-
Dispose of according to local chemical waste regulations.
-
Always refer to the Safety Data Sheet (SDS) before use.
Its stability as a hydrochloride salt means it’s much less volatile and easier to store than many free amines or hydroxylamines.
🧪 Synthesis and Use in the Lab
To synthesize a Weinreb amide, chemists typically react an acyl chloride or carboxylic acid with N,O-Dimethylhydroxylamine in the presence of a base (e.g., triethylamine or pyridine). The resulting amide can then be treated with an organometallic reagent, such as methyl lithium or phenylmagnesium bromide, to give a targeted ketone.
This reaction is widely used in total syntheses of natural products like alkaloids, steroids, and peptides, where functional group compatibility and regioselectivity are vital.
🌍 Environmental and Regulatory Notes
N,O-Dimethylhydroxylamine HCl is not classified as particularly hazardous under most chemical regulatory frameworks (such as REACH or OSHA), but as with all laboratory chemicals, responsible use is critical.
Its low vapor pressure and solid form make it less likely to cause environmental release, and it’s generally considered to have low aquatic toxicity. However, due to its amine and ether functionalities, long-term ecological studies are ongoing.
🧠 Final Thoughts: Why It Matters
The chemistry world is full of flashy molecules, but sometimes it’s the subtle, highly reliable compounds that make the biggest difference. N,O-Dimethylhydroxylamine hydrochloride is one of those unsung heroes.
From ketone synthesis to fine chemical production, DMHA·HCl has earned its place in the chemist’s toolbox. Its combination of stability, selectivity, and versatility makes it a reagent of choice in synthetic chemistry — especially when clean ketone formation is the goal.
Whether you’re a student learning organic synthesis or a seasoned researcher optimizing a drug synthesis route, chances are you’ve crossed paths with this humble but powerful reagent.