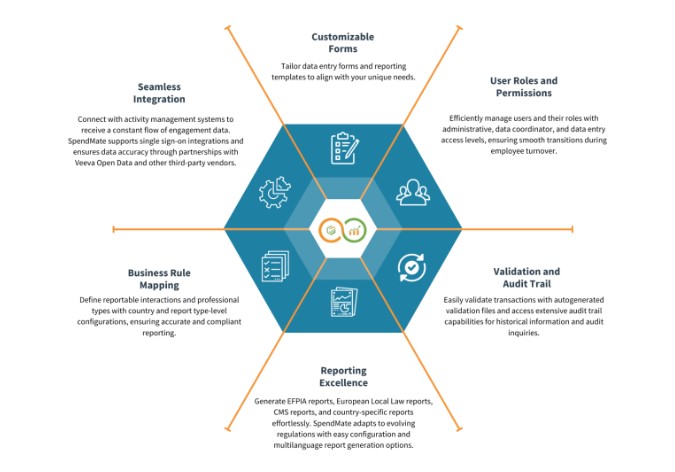
In today’s interconnected world, global compliance transparency reporting has become a critical pillar for businesses, particularly in highly regulated industries like healthcare and pharmaceuticals. With increasing pressure from regulators, consumers, and stakeholders to maintain transparent practices, organizations must adopt strategies that ensure they comply with both global and regional regulations. Among the key components of this reporting landscape are CMS reporting and EFPIA reporting, two essential frameworks for healthcare and pharmaceutical companies to maintain regulatory adherence. These reporting standards help organizations build trust, enhance operational efficiency, and safeguard against legal and reputational risks.
The Rise of Global Compliance Transparency Reporting
As businesses expand their operations internationally, global compliance becomes increasingly complex. Different regions have varying standards and regulatory requirements, creating challenges for organizations striving to maintain consistent transparency across borders. Global compliance transparency reporting refers to the process of adhering to the legal and regulatory requirements related to the disclosure of financial and non-financial data across different jurisdictions. For companies involved in healthcare, life sciences, or pharmaceuticals, this often includes mandatory reporting of transactions and engagements with healthcare professionals (HCPs), institutions, and organizations.
Transparency in financial reporting is essential not just for compliance but for maintaining ethical standards and ensuring that all stakeholders are well-informed. The growing demand for transparency has been driven by global initiatives to combat corruption, incentivize ethical business practices, and protect public health. These requirements are supported by key frameworks like CMS Reporting and EFPIA Reporting, which help healthcare companies comply with both local and global regulations.
Understanding CMS Reporting
The Centers for Medicare & Medicaid Services (CMS), a US-based federal agency, plays a significant role in enforcing healthcare compliance within the United States. One of CMS’s key initiatives is the Physician Payments Sunshine Act (Sunshine Act), which mandates transparency in the financial relationships between pharmaceutical and medical device manufacturers and healthcare providers. Under CMS reporting requirements, healthcare organizations must disclose payments and other transfers of value made to HCPs, physicians, teaching hospitals, and other healthcare entities.
Key Requirements of CMS Reporting
CMS reporting requires healthcare companies to disclose various types of financial arrangements with healthcare providers, including but not limited to:
- Consulting Fees: Payments made to healthcare professionals for their expertise.
- Research Funding: Financial support provided to HCPs for conducting clinical trials or research.
- Gifts and Meals: Reporting gifts, meals, and other items of value provided to healthcare providers.
- Honoraria: Compensation for speakers, advisory board participation, and other professional activities.
- Ownership Interests: Disclosures of any equity or ownership interests held by healthcare providers in a company.
Benefits of CMS Reporting for Healthcare Organizations
Adhering to CMS reporting requirements offers several advantages for healthcare organizations:
- Risk Mitigation: Non-compliance can result in hefty fines, legal actions, and reputational damage. CMS reporting ensures that healthcare organizations stay within legal boundaries.
- Enhanced Transparency: By making financial relationships public, organizations increase transparency, promoting ethical behavior and accountability in healthcare.
- Building Trust: Compliance with CMS reporting demonstrates commitment to ethical conduct and strengthens relationships with patients, providers, and regulatory authorities.
- Avoiding Conflicts of Interest: Full transparency ensures that any potential conflicts of interest are disclosed, minimizing the risk of biased clinical decisions.
The Role of EFPIA Reporting in the Pharmaceutical Industry
The European Federation of Pharmaceutical Industries and Associations (EFPIA) sets out guidelines for pharmaceutical companies operating in Europe to report payments and other transfers of value made to healthcare professionals and organizations. Like CMS, EFPIA reporting is part of the global push toward transparency in the healthcare sector, but it is specifically tailored to European regulations.
EFPIA has implemented a set of requirements that companies must follow when disclosing their interactions with healthcare providers. These requirements aim to ensure that companies’ financial relationships with healthcare professionals are disclosed in a standardized manner across all EU member states.
Key Elements of EFPIA Reporting
EFPIA reporting involves detailed disclosures on various types of financial interactions, including:
- Payments for Services: This includes compensation for consulting, speaking engagements, and advisory board memberships.
- Sponsorship and Research Funding: Companies must disclose financial support for medical research, educational activities, and conferences.
- Non-Financial Benefits: In addition to financial transactions, EFPIA also requires the reporting of non-monetary benefits provided to healthcare professionals and organizations.
- Aggregate Reporting: For some transactions, EFPIA mandates aggregate reporting, where total payments to specific groups or categories of healthcare professionals are disclosed without identifying individual recipients.
Why EFPIA Reporting Matters
- Legal Compliance: Pharmaceutical companies operating in Europe must comply with EFPIA guidelines to avoid legal repercussions. Non-compliance could lead to fines or legal disputes, affecting both profitability and reputation.
- Market Access: Transparent reporting helps companies maintain market access within the European Union. Healthcare providers, institutions, and patients are increasingly scrutinizing the relationships between pharmaceutical companies and medical professionals.
- Public Trust: By adhering to EFPIA reporting, pharmaceutical companies promote a culture of integrity, demonstrating that they are not engaging in unethical practices that could undermine patient care.
- Global Reputation: As global transparency regulations become more interconnected, adherence to EFPIA reporting enhances a company’s reputation on the global stage, signaling its commitment to ethical business practices.
The Intersection of Global Compliance, CMS, and EFPIA Reporting
Both CMS reporting and EFPIA reporting play crucial roles in shaping the broader landscape of global compliance transparency. Healthcare organizations operating across different jurisdictions must ensure that they are not only adhering to local reporting standards but also aligning their practices with international frameworks. The growing complexity of global regulations requires integrated solutions that allow organizations to manage compliance and reporting seamlessly across borders.
Benefits of Integrated Reporting Solutions
- Simplified Reporting Across Borders
An integrated global compliance transparency reporting solution enables healthcare organizations to manage CMS, EFPIA, and other regional reporting requirements from a single platform. This unified approach simplifies the reporting process, reduces errors, and saves time.
- Data Accuracy and Consistency
Automated solutions help ensure that data is accurate and consistent across all reports, regardless of the regulatory framework. With real-time updates and monitoring, healthcare organizations can easily track financial relationships and adjust as needed to remain compliant.
- Cost Efficiency
Instead of maintaining separate reporting systems for each region or country, integrated solutions streamline compliance workflows and reduce overhead costs. Companies can avoid duplicating efforts and focus on core business operations while ensuring full regulatory compliance.
- Faster Time-to-Compliance
Automated tools significantly reduce the time required to prepare and submit reports. This allows healthcare organizations to meet deadlines efficiently and avoid penalties for late submissions.
Conclusion: Embracing Global Compliance Transparency Reporting
In an era of heightened scrutiny and regulatory complexity, global compliance transparency reporting is essential for healthcare organizations and pharmaceutical companies. By embracing frameworks such as CMS reporting and EFPIA reporting, organizations can ensure compliance with local and global regulations while fostering trust and integrity within the industry. The adoption of automated reporting solutions that integrate global and regional requirements provides a strategic advantage in managing compliance efficiently, safeguarding the reputation of the company, and building stronger relationships with stakeholders.